Test Strips: Tips, Techniques, Taboos
This presentation was delivered by Vern Taaffe at the 2009 and 2014 NANT National Symposium in Las Vegas, NV and the 2013 NKF meeting in Deerfield Beach, FL.
Good morning everyone. Thank you for attending this session.
In this session, we will use a three “Ts” approach – tips, techniques, and taboos – to gain a better understanding of the proper use of test strips.
Objectives for the session are:
To describe the various test strips used in dialysis, how they work, and why we use them.
To examine the methods and requirements for proper use of test strips, and
To identify warnings and cautions associated with the use of test strips.
There will be a question and answer period at the end of the session. During that time period, please share any questions you may have.
Copyright RPC - Vern Taaffe, 2008, 2009, 2010, 2011, 2012, 2013, 2014
Good morning everyone. Thank you for attending this session.
In this session, we will use a three “Ts” approach – tips, techniques, and taboos – to gain a better understanding of the proper use of test strips.
Objectives for the session are:
To describe the various test strips used in dialysis, how they work, and why we use them.
To examine the methods and requirements for proper use of test strips, and
To identify warnings and cautions associated with the use of test strips.
There will be a question and answer period at the end of the session. During that time period, please share any questions you may have.
Copyright RPC - Vern Taaffe, 2008, 2009, 2010, 2011, 2012, 2013, 2014

Okay, let’s get started by describing a test strip. What exactly is a test strip?
A test strip is a small plastic strip with a pad or pads attached that has been impregnated with an appropriate amount of reagent for measuring a specific substance in a fluid.
It may also be made from a reagent impregnated paper with no pads attached.
The reagent strip or test strip is a practical and useful tool that has modernized the way laboratory assays are carried out. Its user-friendly qualities allow technical and non-technical persons to conduct relatively sophisticated assays.
A test strip is a small plastic strip with a pad or pads attached that has been impregnated with an appropriate amount of reagent for measuring a specific substance in a fluid.
It may also be made from a reagent impregnated paper with no pads attached.
The reagent strip or test strip is a practical and useful tool that has modernized the way laboratory assays are carried out. Its user-friendly qualities allow technical and non-technical persons to conduct relatively sophisticated assays.
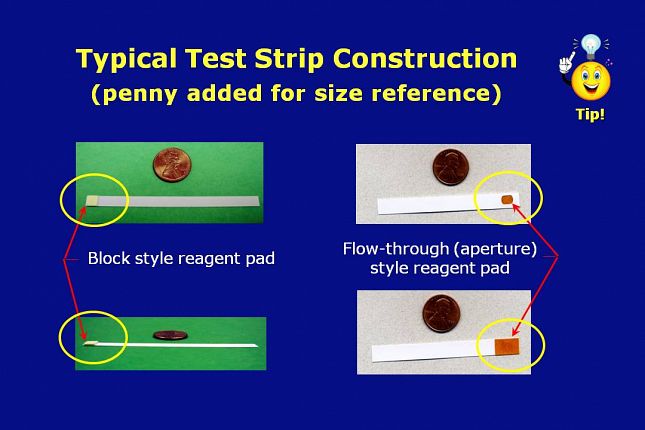
Test strip reagent areas are impregnated with a complex mixture of chemicals that can include enzymes, polymers, surfactants, buffers and indicators. The chemicals control the ability of the reagent area to pick up the sample, enhance the color intensity, provide stability, reduce the tendency of the colors to run off the pad, and enhance other aspects of a test strip.
The strip shown on the left has the reagent pad mounted on top of a solid plastic strip.
The aperture type strip on the right has a hole in the reagent pad mount area of the plastic strip. This allows the test sample to flow-through the pad for increased absorption.
The strip shown on the left has the reagent pad mounted on top of a solid plastic strip.
The aperture type strip on the right has a hole in the reagent pad mount area of the plastic strip. This allows the test sample to flow-through the pad for increased absorption.

Typical test strip packaging includes: • Plastic containers configured as: - Screw cap or flip-top cap bottles with 50 or 100 strips in a bottle - Individual bottles or kits of multiple bottles - Flat packs with a “pull off push on” rectangular cap • Metal tubes having a - “Push off push on” cap that can be used with the CapKeeper® holder - Individual foil-wrapped strips that ship in poly bags
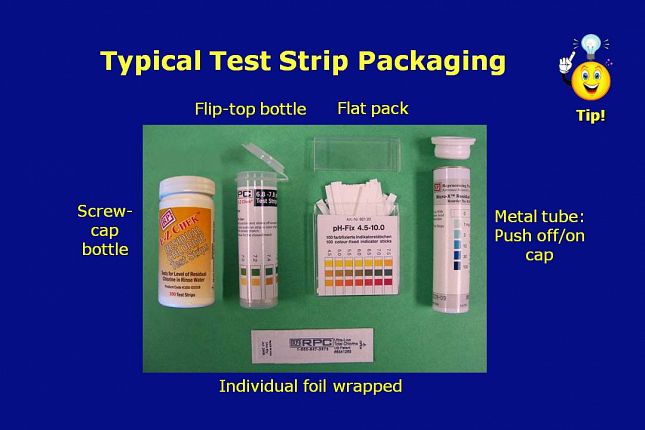
Here is a picture of each of the most common packaging configurations.
The flat pack packaging - shown in the picture as third from the left - is gradually being replaced by the flip-top bottle – shown as second from the left.
The flat pack packaging - shown in the picture as third from the left - is gradually being replaced by the flip-top bottle – shown as second from the left.

Test strips have been used in the medical industry for more than 50 years. The first major breakthrough, for analysis using a test strip format, came with the introduction of CLINISTIX®, a “dip-and-read” test for glucose in urine. It was introduced by Miles Laboratories in the late 1950s. CLINISTIX was the first application for current style test strips in the medical industry.
Shortly after the introduction of test strips for urinalysis came the introduction of test strips for measurement of serum, and subsequently, in the early 1970s, test strips for measurement of chemical parameters in blood.
Today, test strips are used in many applications, including dialysis. They can be used to test feed water, rinse water, water in holding tanks, dialysate pH and practically every other solution that requires testing in the dialysis process.
Shortly after the introduction of test strips for urinalysis came the introduction of test strips for measurement of serum, and subsequently, in the early 1970s, test strips for measurement of chemical parameters in blood.
Today, test strips are used in many applications, including dialysis. They can be used to test feed water, rinse water, water in holding tanks, dialysate pH and practically every other solution that requires testing in the dialysis process.

Why are test strips so well accepted for use in dialysis test applications?
In general, they are an easy to use, fast, and cost effective means of accurately testing dialysis fluids.
In general, they are an easy to use, fast, and cost effective means of accurately testing dialysis fluids.

How do test strips work?
In a typical assay, you dip the reagent area into a sample for a specified time period, remove it, and compare the color of the reagent area with a color chart. The time period to complete a test with a test strip is typically between 5 and 60 seconds.
Some test strips work by indicating the presence or absence of a color change at a threshold concentration, or by measuring a color or electrochemical change with a meter.
Test strips change colors because the presence of the specific parameter in the sample, causes a chemical reaction that leads to a change in the color of one or more of the chemicals in the reagent area of the strip.
In a typical assay, you dip the reagent area into a sample for a specified time period, remove it, and compare the color of the reagent area with a color chart. The time period to complete a test with a test strip is typically between 5 and 60 seconds.
Some test strips work by indicating the presence or absence of a color change at a threshold concentration, or by measuring a color or electrochemical change with a meter.
Test strips change colors because the presence of the specific parameter in the sample, causes a chemical reaction that leads to a change in the color of one or more of the chemicals in the reagent area of the strip.
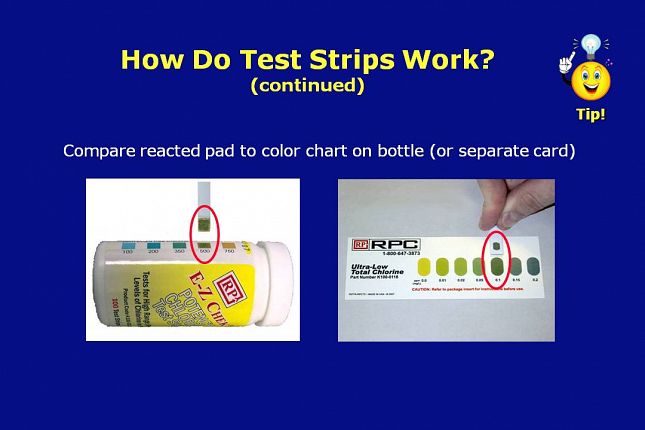
On the left, is a potency chlorine test strip that was tested in a standard reference solution known to have 500 ppm chlorine in water.
The color on the reacted reagent pad properly matches the 500 ppm color block on the bottle label.
On the right, is a total chlorine residual test strip that was tested in a standard reference solution known to have 0.1 ppm chloramine in water.
The color on the reagent pad properly matches the 0.1 ppm color block on the color card packaged with the test strips.
The color on the reacted reagent pad properly matches the 500 ppm color block on the bottle label.
On the right, is a total chlorine residual test strip that was tested in a standard reference solution known to have 0.1 ppm chloramine in water.
The color on the reagent pad properly matches the 0.1 ppm color block on the color card packaged with the test strips.

In 1975, scientists Judd and Wyszecki estimated that there are 10 million definitely distinguishable colors. Color scientists have organized colors into three components: lightness, hue, and saturation. When comparing colors, people with normal color vision can distinguish extremely slight differences in lightness, hue and saturation . A defined standard “color difference unit” is used by color scientists to quantitatively describe differences between colors.
Manufacturers strive to create the greatest possible color difference with concentration in terms of these units. In addition, color measurement tools are used to select the best color match between the test strip and the color blocks that are usually printed on the test strip bottle label or on other packaging materials. The match between reagent strip and color block colors is also checked in different light sources such as fluorescent, daylight and incandescent. This confirms that there is no situation in which the colors appear the same under one viewing condition yet appear different under other viewing conditions.
Manufacturers strive to create the greatest possible color difference with concentration in terms of these units. In addition, color measurement tools are used to select the best color match between the test strip and the color blocks that are usually printed on the test strip bottle label or on other packaging materials. The match between reagent strip and color block colors is also checked in different light sources such as fluorescent, daylight and incandescent. This confirms that there is no situation in which the colors appear the same under one viewing condition yet appear different under other viewing conditions.

Test strips that are commonly used in dialysis centers include:
• Free and Total Chlorine
• Chlorine Potency
• Total Hardness
• Ozone (in water)
• pH
Peroxide/Peracetic Acid Residual
Peracetic Acid Potency
Blood Leak
Glucose (PD Catheter leaks)
• Free and Total Chlorine
• Chlorine Potency
• Total Hardness
• Ozone (in water)
• pH
Peroxide/Peracetic Acid Residual
Peracetic Acid Potency
Blood Leak
Glucose (PD Catheter leaks)
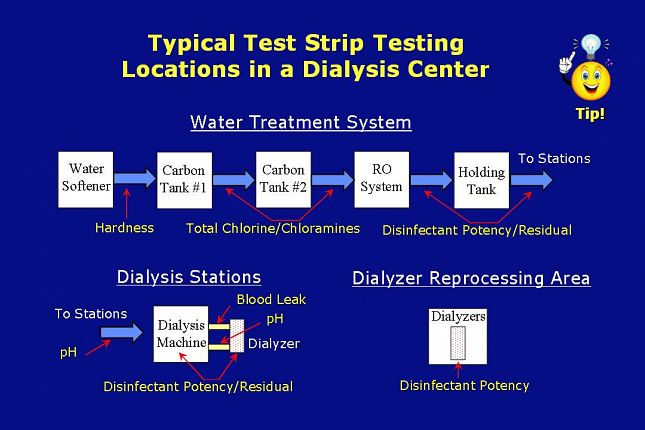
This slide shows a schematic drawing of a representative dialysis operation with typical testing locations in the water treatment area,
the dialysis machine area,
and the dialyzer reprocessing area.
This schematic represents a general example. Your testing routine may vary from that shown here.
the dialysis machine area,
and the dialyzer reprocessing area.
This schematic represents a general example. Your testing routine may vary from that shown here.
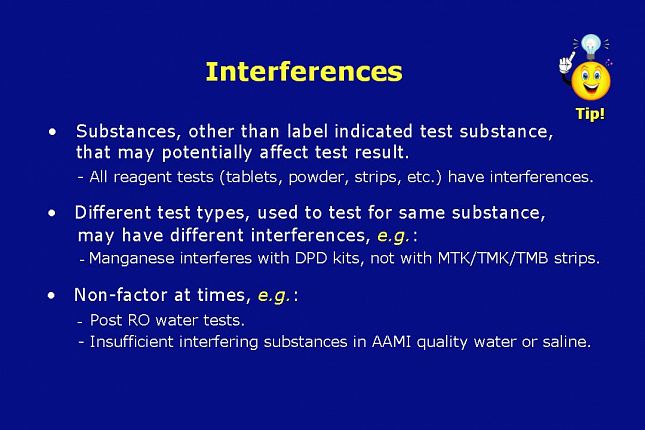
Interferences are substances, other than the label indicated test substance, that may potentially affect the test result. All reagent tests (tablets, powder, strips, etc.) have interferences. Tests based on oxidation technology are likely to be interfered with by oxidizers other than the label indicated test substance. For example: A chlorine test would be expected to be interfered with by peroxide and a peroxide test by chlorine.
Different test types, used to test for the same substance, may have different interferences. For example:
Manganese and certain N-compounds interfere with DPD kit reagents, but not with MTK reagent type total chlorine test strips. This is important to know when performing total chlorine testing between carbon tanks. If the water under test has manganese or N-compounds in it, you may get a false positive with the DPD kit.
Much of the time interferences are a non-factor because tests on, or diluted with, AAMI quality water or diluted with normal saline would not be expected to have interferences.
Nevertheless, be aware of whether or not the test you are performing may be exposed to a potential interfering substance listed in the IFUs for that test.
Different test types, used to test for the same substance, may have different interferences. For example:
Manganese and certain N-compounds interfere with DPD kit reagents, but not with MTK reagent type total chlorine test strips. This is important to know when performing total chlorine testing between carbon tanks. If the water under test has manganese or N-compounds in it, you may get a false positive with the DPD kit.
Much of the time interferences are a non-factor because tests on, or diluted with, AAMI quality water or diluted with normal saline would not be expected to have interferences.
Nevertheless, be aware of whether or not the test you are performing may be exposed to a potential interfering substance listed in the IFUs for that test.

Test strips are comparable in accuracy to liquid color comparator tests and even analytical instruments. They are manufactured and released using standard reference procedures. For example, reagent strip results for chlorine are required to closely match results that are obtained when the same sample is analyzed using amperometric titration per the Standard Method of Wallace and Tiernan.
In addition, “blind studies” are conducted to verify the accuracy. A chemist prepares reference test samples at several different concentrations. People who are unaware of the actual concentrations are asked to assay the test samples with the test strips and report their results. The reported results must correlate with the actual concentrations in order for the product to be released. This also ensures that the reagent strips are consistently reliable from bottle to bottle and lot to lot.
Test strips have fewer end user procedural steps compared to liquid, tablet, and powder test kits and electronic devices.
In addition, “blind studies” are conducted to verify the accuracy. A chemist prepares reference test samples at several different concentrations. People who are unaware of the actual concentrations are asked to assay the test samples with the test strips and report their results. The reported results must correlate with the actual concentrations in order for the product to be released. This also ensures that the reagent strips are consistently reliable from bottle to bottle and lot to lot.
Test strips have fewer end user procedural steps compared to liquid, tablet, and powder test kits and electronic devices.

Are there factors that can affect test strip performance and…
are there steps the end user should take to ensure test strip accuracy?
YES!...Definitely.
There are specific, well-defined steps the end user should take to ensure test strip accuracy.
The factors that affect test strip performance can be controlled by taking the steps outlined on the following slides.
are there steps the end user should take to ensure test strip accuracy?
YES!...Definitely.
There are specific, well-defined steps the end user should take to ensure test strip accuracy.
The factors that affect test strip performance can be controlled by taking the steps outlined on the following slides.
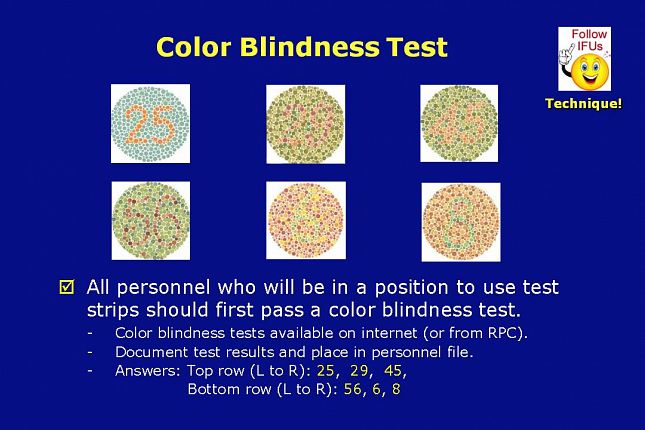
Can you see the numbers inside each circle? It’s important to note here that even with normal color vision, this projector may distort the colors making it difficult to see the numbers.
All personnel who will be in a position to use test strips should first pass a color blindness test.
Color blindness tests are available on the internet or from your test strip vendor.
The test results should be documented and placed in the personnel file.
Answers: Top row (L to R): 25, 29, 45,
Bottom row (L to R): 56, 6, 8
All personnel who will be in a position to use test strips should first pass a color blindness test.
Color blindness tests are available on the internet or from your test strip vendor.
The test results should be documented and placed in the personnel file.
Answers: Top row (L to R): 25, 29, 45,
Bottom row (L to R): 56, 6, 8

Convenience and ease of use of test strips can lead a careless end user to ignore the use instructions for the specific test strip that he or she is using. This can be a major issue, and can lead to incorrect results. There are important use procedures to keep in mind.
These procedures include: Test strip handling, Test sample preparation, Reagent area immersion time and Wetted test strip wait time and the Dip, swish, or flow-over procedure.
These procedures include: Test strip handling, Test sample preparation, Reagent area immersion time and Wetted test strip wait time and the Dip, swish, or flow-over procedure.

In addition to the important test strip procedures, listed on the prior slide, there are key test strip action items that should be considered essential.
These action items include:
• Making use of color interpolation
• Knowing the test substance safe limit/range
• Understanding the “zero” color
• Complying with storage and shelf life requirements
• Complying with test strip quality control requirements
• And sending any suspected failed strips back to the vendor for analysis
These action items include:
• Making use of color interpolation
• Knowing the test substance safe limit/range
• Understanding the “zero” color
• Complying with storage and shelf life requirements
• Complying with test strip quality control requirements
• And sending any suspected failed strips back to the vendor for analysis

Let’s take a look at each of these procedures and action items…starting with Test Strip Handling:
Be sure to keep all unused strips in their original container.
Do not remove the desiccant dryer from the container.
Dry your hands before reaching into the container.
Replace the container cap immediately and tightly after removing a test strip.
Do not touch the indicator (reagent) pad.
and do not allow test strips to come into contact with non-test liquids or any vapors.
Be sure to keep all unused strips in their original container.
Do not remove the desiccant dryer from the container.
Dry your hands before reaching into the container.
Replace the container cap immediately and tightly after removing a test strip.
Do not touch the indicator (reagent) pad.
and do not allow test strips to come into contact with non-test liquids or any vapors.

Be sure to properly prepare the test sample for each specific substance to be tested.
For example:
Prior to testing water:
Allow the RO to process water for at least 15 minutes
If the test calls for the use of a sample cup, rinse the cup three times with the water to be tested.
If you are testing for chlorine, complete the test immediately after preparing the sample as chlorine and chloramines are volatile.
Prior to testing a reprocessed dialyzer for residual peracetic acid:
Carefully follow the germicide manufacturer’s or clinic validated rinse procedure
For example:
Prior to testing water:
Allow the RO to process water for at least 15 minutes
If the test calls for the use of a sample cup, rinse the cup three times with the water to be tested.
If you are testing for chlorine, complete the test immediately after preparing the sample as chlorine and chloramines are volatile.
Prior to testing a reprocessed dialyzer for residual peracetic acid:
Carefully follow the germicide manufacturer’s or clinic validated rinse procedure
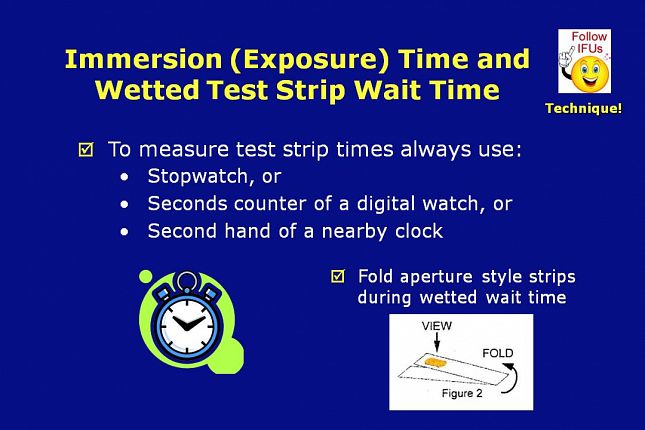
When measuring immersion times or wetted test strip wait times, always use a timing device such as a stopwatch, the seconds counter of a digital watch, or the second hand of a nearby clock.
If called for in the instructions for use, aperture style test strips can be folded in half end-to-end during the wetted test strip wait time. Folding the strip in half, gives better color discernment by placing a white background behind the reagent pad.
Some test strip types require that the results be interpreted immediately after removing the test strip from the fluid sample…and therefore do not have a wetted wait time.
If called for in the instructions for use, aperture style test strips can be folded in half end-to-end during the wetted test strip wait time. Folding the strip in half, gives better color discernment by placing a white background behind the reagent pad.
Some test strip types require that the results be interpreted immediately after removing the test strip from the fluid sample…and therefore do not have a wetted wait time.
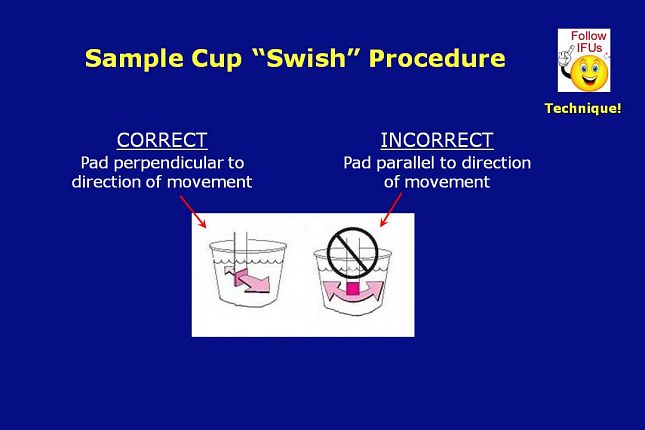
For some test strip types, use of a sample cup is called out in that test strips’ Instructions for Use. When a sample cup is utilized, the instructions for use typically require the testing person to move the test strip back and forth within the sample under test.
When moving or “swishing” the test strip reagent pad in a sample cup, it is very important that the full surface of the pad be moved back and forth in the fluid sample. Moving the thin edge of the strip back and forth causes the sample fluid to flow parallel to the reagent pad and may cause insufficient wetting of the pad.
In procedures where a test strip is held under a fluid stream, make sure the fluid stream flows directly on top of the full surface of the pad, rather than on the thin side or edge of the pad.
When moving or “swishing” the test strip reagent pad in a sample cup, it is very important that the full surface of the pad be moved back and forth in the fluid sample. Moving the thin edge of the strip back and forth causes the sample fluid to flow parallel to the reagent pad and may cause insufficient wetting of the pad.
In procedures where a test strip is held under a fluid stream, make sure the fluid stream flows directly on top of the full surface of the pad, rather than on the thin side or edge of the pad.

Sometimes the sample being tested has a concentration that is not exactly the same as one of the concentration levels printed on the color chart. In that situation, the sample concentration is easily determined by interpolating, or estimating the value, between the nearest color blocks on either side of the activated reagent strip color.
In the picture on the slide, hopefully this computer projector combination allows you to see that the color of the activated reagent pad is clearly less than the 0.1 ppm color and greater than the “zero” color.
The color development could be estimated, or interpolated, as halfway between zero and 0.1 ppm. Therefore the result would be estimated at 50 percent of 0.1 ppm which is 0.05 ppm.
The test strip color chart shown here has good color differentiation between the zero and 0.1 ppm color blocks. Because of the projector distortion, it’s best to look at the actual bottle label to see the color difference.
In the picture on the slide, hopefully this computer projector combination allows you to see that the color of the activated reagent pad is clearly less than the 0.1 ppm color and greater than the “zero” color.
The color development could be estimated, or interpolated, as halfway between zero and 0.1 ppm. Therefore the result would be estimated at 50 percent of 0.1 ppm which is 0.05 ppm.
The test strip color chart shown here has good color differentiation between the zero and 0.1 ppm color blocks. Because of the projector distortion, it’s best to look at the actual bottle label to see the color difference.

For each substance tested, there is a target safe limit, safe range, or safe value that the test result should be compared with.
To ensure patient safety, it is important to be certain of what that limit, range, or value is, for each test you perform.
Three examples are shown on this slide:
For a chloramines in water test, the limit is 0.1 ppm per AAMI RD62.
For dialysate pH, the range is 6.9 to 7.6 per AAMI RD52
For peracetic acid residual, the value must be less than 3 ppm, per the peracetic acid manufacturer’s Instructions for Use.
To ensure patient safety, it is important to be certain of what that limit, range, or value is, for each test you perform.
Three examples are shown on this slide:
For a chloramines in water test, the limit is 0.1 ppm per AAMI RD62.
For dialysate pH, the range is 6.9 to 7.6 per AAMI RD52
For peracetic acid residual, the value must be less than 3 ppm, per the peracetic acid manufacturer’s Instructions for Use.

OK. Here’s a good question for you. What does “zero” color mean?
It means that a reacted reagent pad - matching the color chart “zero” color - indicates the substance under test is below the sensitivity of the test strip and cannot be detected.
It does not mean that the substance level is actually at zero.
It does mean that the substance is at a level less than the lowest color chart value.
For many test strip types, the dry reagent pads direct from the container, may not match the chart zero color. The pads may be a little lighter or darker. This is considered normal.
After the pad is reacted in fluid free of the test substance, typically the pad color changes to more closely match the zero on the color chart.
It means that a reacted reagent pad - matching the color chart “zero” color - indicates the substance under test is below the sensitivity of the test strip and cannot be detected.
It does not mean that the substance level is actually at zero.
It does mean that the substance is at a level less than the lowest color chart value.
For many test strip types, the dry reagent pads direct from the container, may not match the chart zero color. The pads may be a little lighter or darker. This is considered normal.
After the pad is reacted in fluid free of the test substance, typically the pad color changes to more closely match the zero on the color chart.

The effectiveness of all reagent strips and liquid test reagents degrades over time. Dating the product and using it within the specified date and storage conditions is necessary to assure the product performance and quality.
Store test strips in a low humidity environment.
An optimal environment would have less than 50% relative humidity at a standard room temperature between 70 and 75 degrees Fahrenheit. A temperature range of 59°-86°F is acceptable for most test strips. However, be sure to check the instructions for use for each different test strip type that you are using in your clinic.
Keep the container cap sealed tightly. Do not remove the desiccant dryer from the test strip container.
Store test strips in a low humidity environment.
An optimal environment would have less than 50% relative humidity at a standard room temperature between 70 and 75 degrees Fahrenheit. A temperature range of 59°-86°F is acceptable for most test strips. However, be sure to check the instructions for use for each different test strip type that you are using in your clinic.
Keep the container cap sealed tightly. Do not remove the desiccant dryer from the test strip container.
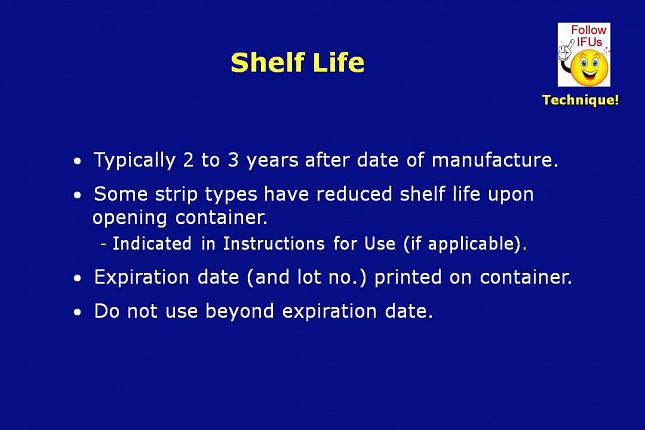
Shelf life is typically 2 to 3 years after the date of manufacture.
- Some vendor’s test strips have a reduced shelf life upon opening the container.
This is indicated in the Instructions for Use if applicable.
- The test strip lot number and expiration date are printed on each container.
The expiration date takes into consideration minor degradation that may occur as a
result of limited exposure to temperature extremes during shipment of the strips.
- Do not use any test strips beyond their expiration date.
- Some vendor’s test strips have a reduced shelf life upon opening the container.
This is indicated in the Instructions for Use if applicable.
- The test strip lot number and expiration date are printed on each container.
The expiration date takes into consideration minor degradation that may occur as a
result of limited exposure to temperature extremes during shipment of the strips.
- Do not use any test strips beyond their expiration date.

Test strip instructions for use often call for verification of test strips through the use of QC test supplies. The QC test supplies may be packaged in with the test strip kits, or they may be ordered separately, for use in test strip verification checks at the clinic.
Documentation of QC verification for test strips is required by CMS.
An option to performing the test strip QC verification in your clinic is a QC Control Verification Program offered by one of the test strip manufacturers. For all test strips shipped from that manufacturer, the dialysis verification is performed for you. Certi-Chek® is the name of the program.
Lot-by-lot test results can be downloaded from the manufacturer’s Web site.
Test result documents downloaded from their Web site are accepted by CMS
It’s also important to note that this secondary lot verification program can help protect against test
strip recalls.
OK. We’ve covered test strip tips and techniques. On the next few slides let’s take a look at the test strip “taboos”.
• Do not use any test strips beyond their expiration date.
Documentation of QC verification for test strips is required by CMS.
An option to performing the test strip QC verification in your clinic is a QC Control Verification Program offered by one of the test strip manufacturers. For all test strips shipped from that manufacturer, the dialysis verification is performed for you. Certi-Chek® is the name of the program.
Lot-by-lot test results can be downloaded from the manufacturer’s Web site.
Test result documents downloaded from their Web site are accepted by CMS
It’s also important to note that this secondary lot verification program can help protect against test
strip recalls.
OK. We’ve covered test strip tips and techniques. On the next few slides let’s take a look at the test strip “taboos”.
• Do not use any test strips beyond their expiration date.

Do not expect tap water chlorine tests to be consistent or uniform.
Levels of chlorine/chloramines, from tap water faucets in the same building, can vary because chlorine is volatile and the level is affected by piping materials and other factors.
In addition, the raw water provider may allow the level of chlorine/chloramines to vary anywhere within the EPA range of 0.2 ppm (minimum) to 4.0 ppm (maximum).
Expectation that a total chlorine test strip should always show a relatively consistent 1, 2, or 3 ppm chlorine value in tap water is not realistic.
Levels of chlorine/chloramines, from tap water faucets in the same building, can vary because chlorine is volatile and the level is affected by piping materials and other factors.
In addition, the raw water provider may allow the level of chlorine/chloramines to vary anywhere within the EPA range of 0.2 ppm (minimum) to 4.0 ppm (maximum).
Expectation that a total chlorine test strip should always show a relatively consistent 1, 2, or 3 ppm chlorine value in tap water is not realistic.

Do not use a qualitative test for tests requiring low end precision.
Qualitative & semi-quantitative procedures may both be listed in the test strip Instructions for Use.
At the lowest measurement value or color block, the precision of the qualitative test may be affected by the speed of the sample flow.
Precision is defined as repeatability, or the ability to repeat the test with consistent results.
Qualitative & semi-quantitative procedures may both be listed in the test strip Instructions for Use.
At the lowest measurement value or color block, the precision of the qualitative test may be affected by the speed of the sample flow.
Precision is defined as repeatability, or the ability to repeat the test with consistent results.

Here are some additional helpful definitions.
A chemical qualitative analysis determines the constituents of a substance without regard to the quantity of each ingredient. [1913 Webster]. Said another way it is a type of presence or indicator test.
A chemical quantitative analysis determines the amount or quantity of each ingredient of a substance. [1913 Webster]
A chemical analysis is the separation of a compound substance, by chemical processes, into its constituents, with a view to determine either (a) what elements it contains, or (b) how much of each element is present. The former is called qualitative, and the latter quantitative analysis. [1913 Webster]
A chemical qualitative analysis determines the constituents of a substance without regard to the quantity of each ingredient. [1913 Webster]. Said another way it is a type of presence or indicator test.
A chemical quantitative analysis determines the amount or quantity of each ingredient of a substance. [1913 Webster]
A chemical analysis is the separation of a compound substance, by chemical processes, into its constituents, with a view to determine either (a) what elements it contains, or (b) how much of each element is present. The former is called qualitative, and the latter quantitative analysis. [1913 Webster]

This slide gives an example of proper application of the qualitative and quantitative test procedures when used with Total Chlorine Sensitive Test Strips.
Some total chlorine sensitive test strips list both qualitative and quantitative
procedures in the instructions for use.
The qualitative procedure can be used for checking chlorine rinse residuals in
water distribution loops, jugs, and dialysis machines or wherever a 0.5 ppm
chlorine detection level is appropriate.
Always use the quantitative procedure for sensitive tests such as tests between carbon tanks where the total chlorine must not exceed 0.1 ppm.
For some test strip procedures the sample volume is not critical; yet for other test strip procedures the sample volume is critical. Quantitative tests call for a specific test sample volume. To eliminate any possibility of sample size error, always use the exact sample volume called out in the test strip’s Instructions For Use.
Some total chlorine sensitive test strips list both qualitative and quantitative
procedures in the instructions for use.
The qualitative procedure can be used for checking chlorine rinse residuals in
water distribution loops, jugs, and dialysis machines or wherever a 0.5 ppm
chlorine detection level is appropriate.
Always use the quantitative procedure for sensitive tests such as tests between carbon tanks where the total chlorine must not exceed 0.1 ppm.
For some test strip procedures the sample volume is not critical; yet for other test strip procedures the sample volume is critical. Quantitative tests call for a specific test sample volume. To eliminate any possibility of sample size error, always use the exact sample volume called out in the test strip’s Instructions For Use.
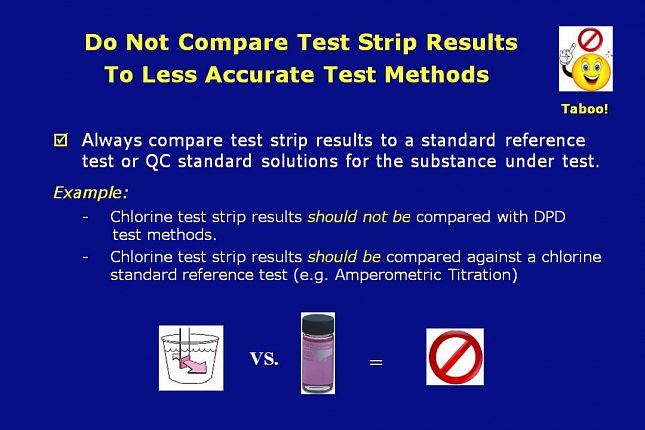
Do not compare test strip results to less accurate test methods.
Always compare test strip results to a standard reference test or a QC standard solution for the substance under test.
For example:
Chlorine test strip results should not be compared with DPD test methods.
Chlorine test strip results should be compared against a chlorine standard reference test such as amperometric titration or a fresh standard solution - of a known value - that is traceable to a standard reference test.
Always compare test strip results to a standard reference test or a QC standard solution for the substance under test.
For example:
Chlorine test strip results should not be compared with DPD test methods.
Chlorine test strip results should be compared against a chlorine standard reference test such as amperometric titration or a fresh standard solution - of a known value - that is traceable to a standard reference test.

Do not use test strips that show discoloration direct from the container.
Reagent test pads direct from the container do not need to match the color chart zero color. A little lighter or darker color is acceptable. The test pad color must match the chart zero color when reacted in water known to be free of the test substance and any interfering substances.
The reagent pad color should be uniform, not “spotty”.
If the pad is irregular brown or black, or spotted, do not use the test strips from that container.
Discoloration of this nature typically means the strips were exposed to excessive moisture and/or heat. Return the test strips to the vendor for analysis.
Reagent test pads direct from the container do not need to match the color chart zero color. A little lighter or darker color is acceptable. The test pad color must match the chart zero color when reacted in water known to be free of the test substance and any interfering substances.
The reagent pad color should be uniform, not “spotty”.
If the pad is irregular brown or black, or spotted, do not use the test strips from that container.
Discoloration of this nature typically means the strips were exposed to excessive moisture and/or heat. Return the test strips to the vendor for analysis.

Bottle labels and/or color charts are specially made to show the same color for each color circle/block under various lighting conditions.
Laminating, covering with film, or modifying the label or color chart in any way may cause the color circles/blocks to show a different color than that intended by the manufacturer…
and this may result in inaccurate test readings.
Laminating, covering with film, or modifying the label or color chart in any way may cause the color circles/blocks to show a different color than that intended by the manufacturer…
and this may result in inaccurate test readings.

To summarize:
Test strips are in widespread use in the medical industry, including dialysis.
They are a fast, convenient, and accurate test method when used properly.
As with other tests - to ensure accuracy and repeatability - it is very important that the specific instructions for use for each test strip be followed carefully.
And last but not least, it is necessary to avoid testing traps or “Taboos” that can cause problems.
How many of you are familiar with the dialysis technician forum on the RenalWeb website? You are probably aware that in the past there have been many pages of blog entries regarding issues with chlorine testing between carbon tanks using DPD based colorimeters. The fact that these blog entries have essentially been eliminated consistent with the U.S. dialysis industry’s change to test strips for this test, is clear evidence of the effectiveness of test strips.
Test strips are in widespread use in the medical industry, including dialysis.
They are a fast, convenient, and accurate test method when used properly.
As with other tests - to ensure accuracy and repeatability - it is very important that the specific instructions for use for each test strip be followed carefully.
And last but not least, it is necessary to avoid testing traps or “Taboos” that can cause problems.
How many of you are familiar with the dialysis technician forum on the RenalWeb website? You are probably aware that in the past there have been many pages of blog entries regarding issues with chlorine testing between carbon tanks using DPD based colorimeters. The fact that these blog entries have essentially been eliminated consistent with the U.S. dialysis industry’s change to test strips for this test, is clear evidence of the effectiveness of test strips.

Let’s end this session on a few notes of interest:
The AAMI Renal Disease and Detoxification Committee is working on a Technical Information Report, or TIR, on test methods used for dialysis water. The TIR will include information on test strips. It is in it’s final review stage and should be released later this year.
CMS has been using this presentation for ESRD surveyor training. To keep up with CMS, you may want to use the presentation for a training tool within your dialysis clinic’s overall documented process control procedures. “Documented process control” is the answer to the following question often asked by ESRD surveyors:
“How do you know these test strips or test instruments will work properly?”
This presentation, and more educational information, can be found in the “Technical Support Information” section of the RPC Web site at the address shown on the slide.
Thank you very much for your kind attention.
At this time feel free to ask any questions you may have.
The AAMI Renal Disease and Detoxification Committee is working on a Technical Information Report, or TIR, on test methods used for dialysis water. The TIR will include information on test strips. It is in it’s final review stage and should be released later this year.
CMS has been using this presentation for ESRD surveyor training. To keep up with CMS, you may want to use the presentation for a training tool within your dialysis clinic’s overall documented process control procedures. “Documented process control” is the answer to the following question often asked by ESRD surveyors:
“How do you know these test strips or test instruments will work properly?”
This presentation, and more educational information, can be found in the “Technical Support Information” section of the RPC Web site at the address shown on the slide.
Thank you very much for your kind attention.
At this time feel free to ask any questions you may have.
