Dialysis Devices: A Photographic History of Progress
Download (.zip)
Good morning everyone ... thank you for attending this session.
For those of you who are pioneers in the industry, or as some might say ... dialysis dinosaurs, this session will represent a trip down memory lane. For others, I hope it will be an interesting and educational overview on the history of dialysis devices.
Please hold your questions until all the slides have been shown.
At that time, we can have an interactive discussion by navigating to any particular slide associated with the questions or comments that you present.
Copyright © RPC - Vern Taaffe (2011)
For those of you who are pioneers in the industry, or as some might say ... dialysis dinosaurs, this session will represent a trip down memory lane. For others, I hope it will be an interesting and educational overview on the history of dialysis devices.
Please hold your questions until all the slides have been shown.
At that time, we can have an interactive discussion by navigating to any particular slide associated with the questions or comments that you present.
Copyright © RPC - Vern Taaffe (2011)

This slide lists the different dialysis devices we will cover today.
In this presentation, for the most part, I’ve tried to include those devices that made the greatest impact and were ... or are ... well known. It was quite a task finding pictures for each of the devices ... and every slide from this point on will have one or more pictures.
After we have completed the slides, please forgive me if I have not included a specific device that you think is important. Feel free to comment on any such device during our Q&A period.
Date references on the slides were verified using various resources including the FDA 510k database for medical devices. A plus sign next to a date means that the device continued in service for a number of years beyond its introduction date.
In this presentation, for the most part, I’ve tried to include those devices that made the greatest impact and were ... or are ... well known. It was quite a task finding pictures for each of the devices ... and every slide from this point on will have one or more pictures.
After we have completed the slides, please forgive me if I have not included a specific device that you think is important. Feel free to comment on any such device during our Q&A period.
Date references on the slides were verified using various resources including the FDA 510k database for medical devices. A plus sign next to a date means that the device continued in service for a number of years beyond its introduction date.

Anytime a history of dialysis devices is presented, the late Dr. Willem Kolff should be mentioned as the person credited with the invention of the artificial kidney. Dr. Kolff passed away in 2009 at the age of 97.
In this picture, Dr. Kolff is shown with my good friend George Rovegno, who is a dialysis pioneer in his own right.
Many of you may know George. He is the CEO of MIQS, a dialysis software company in Boulder, Colorado. George was one of the speakers at the 2010 NANT symposium.
Okay. Let’s get started by taking a look at dialyzers.
In this picture, Dr. Kolff is shown with my good friend George Rovegno, who is a dialysis pioneer in his own right.
Many of you may know George. He is the CEO of MIQS, a dialysis software company in Boulder, Colorado. George was one of the speakers at the 2010 NANT symposium.
Okay. Let’s get started by taking a look at dialyzers.

The first hemodialyzer was the rotating drum dialyzer invented by Willem Kolff in 1943.
A few years later, at Western Reserve University in Ohio, Leonard Skeggs and Jack Leonards developed the first parallel plate dialyzer.
The Skeggs-Leonards device had a very low resistance to blood flow and it could be used without a blood pump. It had multiple layers and required a great deal of time to construct. Fluid could be removed from the blood by creating a siphon on the dialysate effluent. It is likely that this is the first reference to negative pressure dialysis.
A few years later, at Western Reserve University in Ohio, Leonard Skeggs and Jack Leonards developed the first parallel plate dialyzer.
The Skeggs-Leonards device had a very low resistance to blood flow and it could be used without a blood pump. It had multiple layers and required a great deal of time to construct. Fluid could be removed from the blood by creating a siphon on the dialysate effluent. It is likely that this is the first reference to negative pressure dialysis.
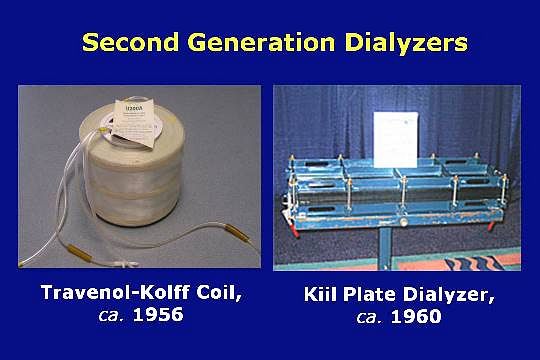
In 1956, with development help from Willem Kolff, Travenol a division of Baxter, introduced a twin coil dialyzer. It was the first commercially available and completely disposable dialyzer.
In 1960 the Kiil plate dialyzer was introduced.
The Kiil dialyzers were produced in Norway. The priming volume was less than 300 mL. When a shunt access was used, the blood could be pumped through the device with blood pressure only; no blood pump was needed. Excess fluid was removed by the use of negative pressure on the dialysate effluent line.
In 1960 the Kiil plate dialyzer was introduced.
The Kiil dialyzers were produced in Norway. The priming volume was less than 300 mL. When a shunt access was used, the blood could be pumped through the device with blood pressure only; no blood pump was needed. Excess fluid was removed by the use of negative pressure on the dialysate effluent line.

In 1964, a major step forward was the development of the hollow-fiber dialyzer by the American Richard Stewart. This technology replaced the until-then traditional membranous tubes and flat membranes with a number of capillary-sized hollow fiber membranes. This procedure allows for the production of dialyzers with a surface area large enough to fulfill the demands of efficient dialysis treatment.
The development of the related industrial manufacturing technology was completed by Dow Chemical between 1964 and 1967. In subsequent years, this new technology allowed the production of large numbers of dialyzers at a reasonable price. The typical hollow-fiber dialyzers of today – which are equipped with a more effective and better-tolerated membrane made primarily from synthetic polymers – are still based on these concepts.
The development of the related industrial manufacturing technology was completed by Dow Chemical between 1964 and 1967. In subsequent years, this new technology allowed the production of large numbers of dialyzers at a reasonable price. The typical hollow-fiber dialyzers of today – which are equipped with a more effective and better-tolerated membrane made primarily from synthetic polymers – are still based on these concepts.
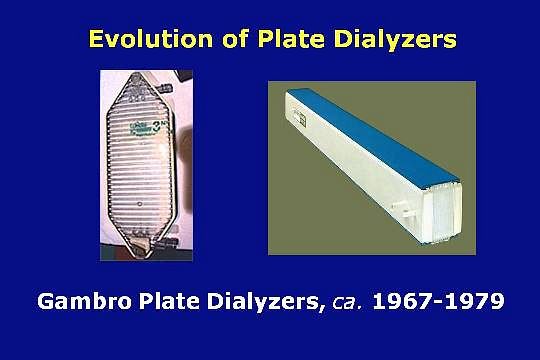
In the years 1967 through 1979, Gambro played an important role by producing parallel plate dialyzers having a lower blood volume, reduced resistance to flow, and a lower cost.
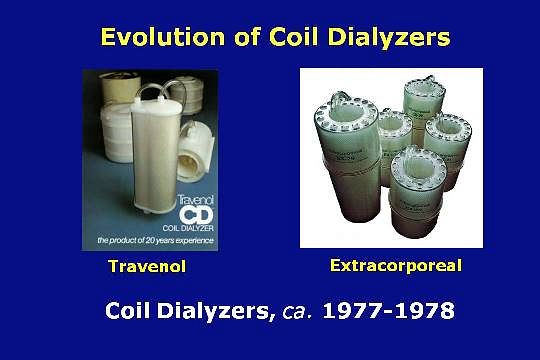
These dialyzers represented the last generation of 20 years of coil development. They used a Cuprophan membrane. Both had clear cases to make it easy to see into the dialyzer during blood rinse back.
In addition, having both dialysate ports on the bottom reduced splashing in the dialyzing compartment.
In addition, having both dialysate ports on the bottom reduced splashing in the dialyzing compartment.

This generation of hollow fiber dialyzers first appeared in the late 1970s. They were very popular for many years after that. During the early 1980s, when I was with Renal Systems, these dialyzers were among the first we used during the development of the original Renatron. All three shown here were excellent dialyzers for reprocessing.

In the mid-1980s Baxter introduced a series of high efficiency and high flux dialyzers. Fresenius also introduced a series of high flux dialyzers.
Like it was yesterday, I remember receiving a call from my good friend Steve Lauer at the Regional Kidney Disease Program in Minneapolis. Steve called to let me know that the Baxter high efficiency and high flux dialyzers did not reprocess properly on the Renatron. It was at that point that I developed different software programs for the Renatron to accommodate the more permeable high efficiency and high flux dialyzers.
These programs were integrated into an updated version of the Renatron that had selectable reprocessing programs.
Many thanks to Steve for the time and effort he put in to help validate the high efficiency and high flux programs.
Like it was yesterday, I remember receiving a call from my good friend Steve Lauer at the Regional Kidney Disease Program in Minneapolis. Steve called to let me know that the Baxter high efficiency and high flux dialyzers did not reprocess properly on the Renatron. It was at that point that I developed different software programs for the Renatron to accommodate the more permeable high efficiency and high flux dialyzers.
These programs were integrated into an updated version of the Renatron that had selectable reprocessing programs.
Many thanks to Steve for the time and effort he put in to help validate the high efficiency and high flux programs.

More recent dialyzers used today include the Gambro Revaclear, Baxter Xenium, and Fresenius Optiflux.

When reviewing the path of dialyzer progress, I think its important to recognize that there have been bumps in the road along the way.
In the Fall of 2001, there were 53 sudden patient deaths attributed to the use of the Althane series of Althin/Baxter dialyzers. Residuals from a PF-5070 perfluorocarbon agent, used for testing leaking dialyzers, remained in the dialyzers at the initiation of dialysis. As the dialyzers were heated to body temperature, the PF-5070 agent changed from liquid to gas and caused foam embolisms in each of the patients. How many of you remember this incident?
It is important to note that in all 53 cases the dialyzer was a new dialyzer that had not been pre-processed as a part of a good reuse program. The PF-5070 data and MSDS sheets make it clear that the residual agent would have been rinsed from the dialyzers had they been pre-processed for reuse.
This of course makes a strong case for reuse as a medical benefit.
In the Fall of 2001, there were 53 sudden patient deaths attributed to the use of the Althane series of Althin/Baxter dialyzers. Residuals from a PF-5070 perfluorocarbon agent, used for testing leaking dialyzers, remained in the dialyzers at the initiation of dialysis. As the dialyzers were heated to body temperature, the PF-5070 agent changed from liquid to gas and caused foam embolisms in each of the patients. How many of you remember this incident?
It is important to note that in all 53 cases the dialyzer was a new dialyzer that had not been pre-processed as a part of a good reuse program. The PF-5070 data and MSDS sheets make it clear that the residual agent would have been rinsed from the dialyzers had they been pre-processed for reuse.
This of course makes a strong case for reuse as a medical benefit.

Okay. Let’s move on from dialyzers to dialysis machines.
The Travenol RSP, shown on the left, was initially used with coil dialyzers and it had a batch tank. The machine required that the dialysate bath be mixed in the batch tank each time. The batch tank contained 120 liters of water and concentrate. Many centers used ordinary tap water.
So that hollow fiber dialyzers could be used with it, ultimately Travenol introduced a negative pressure converter for the RSP.
The Drake-Willock 4000 series were the first machines to contain a proportioning pump. This eliminated the need for a dialysate batch tank and made automatic on-line dialysate mixing possible.
For delivering dialysate to multiple patient stations, central batch systems and central proportioning systems were also popular in the 1960s and beyond.
The Travenol RSP, shown on the left, was initially used with coil dialyzers and it had a batch tank. The machine required that the dialysate bath be mixed in the batch tank each time. The batch tank contained 120 liters of water and concentrate. Many centers used ordinary tap water.
So that hollow fiber dialyzers could be used with it, ultimately Travenol introduced a negative pressure converter for the RSP.
The Drake-Willock 4000 series were the first machines to contain a proportioning pump. This eliminated the need for a dialysate batch tank and made automatic on-line dialysate mixing possible.
For delivering dialysate to multiple patient stations, central batch systems and central proportioning systems were also popular in the 1960s and beyond.

The Model A shown on the left was built by the Milton Roy Company in St. Petersburg, Florida in 1964. It was designed to perform nocturnal home hemodialysis. It had a wooden veneer cabinet that gave it a furniture appearance for the home. It featured automatic hot water disinfection, automatic alarm checks, solid-state logic, and acoustic tile inside to reduce noise.
Because Milton Roy formed a joint venture with Extracorporeal in 1973, and in 1984 Baxter acquired the dialysis equipment division of Extracorporeal, the Milton Roy machines shown here were the predecessors to the Extracorporeal and Baxter dialysis machines.
The Model A machine became the first of a series of negative pressure single patient systems culminating in the 9th generation, Baxter Arena machine introduced in 2003.
Because Milton Roy formed a joint venture with Extracorporeal in 1973, and in 1984 Baxter acquired the dialysis equipment division of Extracorporeal, the Milton Roy machines shown here were the predecessors to the Extracorporeal and Baxter dialysis machines.
The Model A machine became the first of a series of negative pressure single patient systems culminating in the 9th generation, Baxter Arena machine introduced in 2003.

In the last two slides, I labeled the machines as dialysate delivery systems. That was the correct terminology for a machine without the standalone ancillary items such as a blood pump, pressure monitor, and air detector.
This slide shows some of the more popular devices used with the delivery systems. The photo in the top left corner shows the dialysate meter being used to test the dialysate conductivity in the batch tank of a Travenol RSP machine. It was not uncommon for the person performing the test to accidentally drop the meter into the dialysate, resulting in the destruction of the meter circuit board.
The photo in the top left corner also shows the huge Travenol blood pump sitting on the RSP shelf. If you look closely at the top left side of the pump you can also see a Travenol pressure monitor.
This slide shows some of the more popular devices used with the delivery systems. The photo in the top left corner shows the dialysate meter being used to test the dialysate conductivity in the batch tank of a Travenol RSP machine. It was not uncommon for the person performing the test to accidentally drop the meter into the dialysate, resulting in the destruction of the meter circuit board.
The photo in the top left corner also shows the huge Travenol blood pump sitting on the RSP shelf. If you look closely at the top left side of the pump you can also see a Travenol pressure monitor.
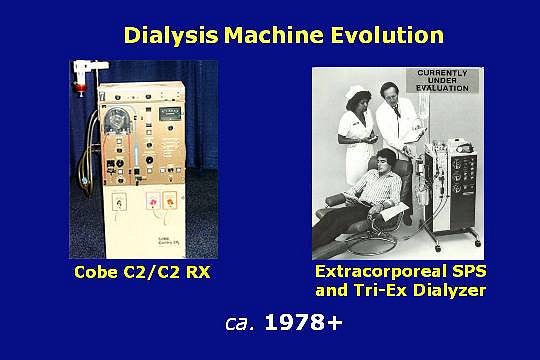
The Cobe Centry Systems were the first servo controlled dialysis machines and the Cobe C2 shown here was the first fully integrated dialysis machine. The blood pump, conductivity monitor, pressure monitors, and air detector were all built into the C2.
Extracorporeal’s Single Patient System, like the Cobe C2, was fully integrated. In the United States, these complete and popular dialysis machines quickly replaced the majority of central dialysate delivery systems.
Extracorporeal’s Single Patient System, like the Cobe C2, was fully integrated. In the United States, these complete and popular dialysis machines quickly replaced the majority of central dialysate delivery systems.

Cordis Dow’s Seratron machine was released in 1979. To promote the machine, I remember that Cordis put a Seratron into a large motor-home type vehicle and drove it to dialysis clinics across the country. Their promotional efforts were disrupted by technical problems with the machine ... and it did not do well.
In regard to the AK-10, Gambro claims that it was the first computer-controlled dialysis machine. The AK-10 was in widespread use in the United States and other countries.
In 1982, Ben Lipps left Cordis Dow and founded Seratronics, Incorporated. Seratronics was known for its DRS-4 dialyzer reprocessing system. However, Seratronics eventually became much better known after it began selling the German made Fresenius dialysis machines, and in 1987 Seratronics merged with Fresenius to become Fresenius USA.
In regard to the AK-10, Gambro claims that it was the first computer-controlled dialysis machine. The AK-10 was in widespread use in the United States and other countries.
In 1982, Ben Lipps left Cordis Dow and founded Seratronics, Incorporated. Seratronics was known for its DRS-4 dialyzer reprocessing system. However, Seratronics eventually became much better known after it began selling the German made Fresenius dialysis machines, and in 1987 Seratronics merged with Fresenius to become Fresenius USA.

After Baxter acquired Extracorporeal’s dialysis equipment division in 1984, the evolution of the popular Extracorporeal SPS machines continued with the release of the Baxter 550 and ultimately the release of the 1550 version.
During this time period, the Fresenius machines had evolved from the 2008A to the 2008E version.
During this time period, the Fresenius machines had evolved from the 2008A to the 2008E version.
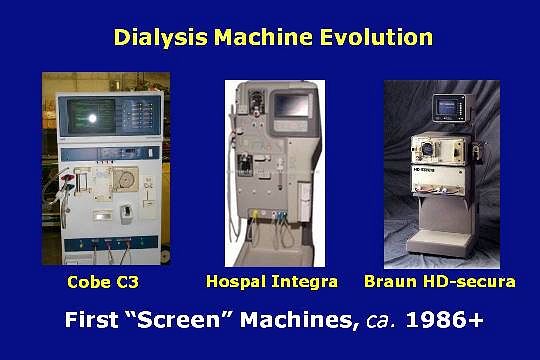
In the latter part of the 1980s, the first screen type machines appeared with the introduction of the Cobe C3, Hospal Integra, and B. Braun HD-secura.
In 1987, Gambro acquired Hospal ... and in 1990 Gambro acquired Cobe. These acquisitions resulted in Gambro having to “juggle” multiple dialysis machines without a promotional focus on any one specific machine.
In 1987, Gambro acquired Hospal ... and in 1990 Gambro acquired Cobe. These acquisitions resulted in Gambro having to “juggle” multiple dialysis machines without a promotional focus on any one specific machine.

In the mid-1980s CD Medical acquired Drake-Willock from Becton Dickenson. In 1991, the Drake-Willock division of CD medical introduced the System 1000. It won awards for its stylish appearance. The System 1000 was one of the first, if not the very first, machine with a touch screen.
In 1990, Althin Medical, a Swedish company acquired CD Medical, the Miami Lakes, Florida based manufacturer of the Cordis-Dow products. Althin’s plant in Ronneby, Sweden manufactured the tainted Althane dialyzers referred to earlier.
Also in the 1990s, Althin moved the Drake dialysis machine manufacturing from Portland, Oregon, to its US headquarters in Miami Lakes, Florida.
In 1997, Althin introduced an updated version of the System 1000 and named it “Tina”. In the year 2000, Baxter acquired Althin Medical.
In 1990, Althin Medical, a Swedish company acquired CD Medical, the Miami Lakes, Florida based manufacturer of the Cordis-Dow products. Althin’s plant in Ronneby, Sweden manufactured the tainted Althane dialyzers referred to earlier.
Also in the 1990s, Althin moved the Drake dialysis machine manufacturing from Portland, Oregon, to its US headquarters in Miami Lakes, Florida.
In 1997, Althin introduced an updated version of the System 1000 and named it “Tina”. In the year 2000, Baxter acquired Althin Medical.

With the introduction of its own dialysis machines shown here, and the addition of the Althin machines, Baxter now faced a similar situation to what Gambro encountered after acquiring Hospal and Cobe.
While these acquisitions reduced the number of dialysis machine manufacturers in the marketplace, the need to manage and support multiple types of existing dialysis machines, detracted from Gambro and Baxter’s ability to focus on and promote one specific line of machines.
Do you think that this could have been a major factor in the decline from prominence for Gambro and Baxter, in the U.S. dialysis machine market?
While these acquisitions reduced the number of dialysis machine manufacturers in the marketplace, the need to manage and support multiple types of existing dialysis machines, detracted from Gambro and Baxter’s ability to focus on and promote one specific line of machines.
Do you think that this could have been a major factor in the decline from prominence for Gambro and Baxter, in the U.S. dialysis machine market?

In 1996, Fresenius announced that it was going to spin off their own dialysis division and merge it with its U.S. subsidiary, Fresenius USA, and also with National Medical Care, to create a new company called Fresenius Medical Care. The transaction created the world’s largest vertically-integrated company in the dialysis market. Fresenius would provide the products needed to treat some 43,000 patients in NMC’s roughly 600 dialysis centers across the United States.
Before the deal, Fresenius had provided only one-third of NMC’s dialysis products purchases. The merger led to a dominant position for Fresenius for dialysis machines and other dialysis products in the U.S. market. The B. Braun Dialog and Gambro Phoenix machines shown here compete with the Fresenius machine; however, in 2010 their number of machines placed in the United States is small in comparison to Fresenius. This is not to say that these machines are not as good as the Fresenius machine. It is only an indication of the dominant position Fresenius enjoys as a result of owning such a large number of dialysis clinics in the United States.
Before the deal, Fresenius had provided only one-third of NMC’s dialysis products purchases. The merger led to a dominant position for Fresenius for dialysis machines and other dialysis products in the U.S. market. The B. Braun Dialog and Gambro Phoenix machines shown here compete with the Fresenius machine; however, in 2010 their number of machines placed in the United States is small in comparison to Fresenius. This is not to say that these machines are not as good as the Fresenius machine. It is only an indication of the dominant position Fresenius enjoys as a result of owning such a large number of dialysis clinics in the United States.

Late last year (2010), Fresenius introduced the 2008T dialysis machine. The picture shown here is from a press release published by Fresenius in November of last year.
In its FDA 510k summary for the 2008T, Fresenius made the following statement:
The Fresenius modified 2008T hemodialysis machine incorporates changes with regard to the user interface only ... and all water requirements, module options, functional options, performance limits, control parameters, compatible bloodlines, and language options remain unchanged from the predicate device, the 2008K.
Also, an article in the February 2011 issue of Renal Business Today takes “A Peek Inside the New Fresenius 2008T”. From the article it’s clear that the change to the user interface refers to the addition of an integrated medical information system that will work with the software from any of the major vendors in the dialysis MIS industry.
Recent feedback on RenalWeb suggests that the 2008T is now readily available for trial or purchase. How many of you here have conducted trials with this machine in your dialysis center?
In its FDA 510k summary for the 2008T, Fresenius made the following statement:
The Fresenius modified 2008T hemodialysis machine incorporates changes with regard to the user interface only ... and all water requirements, module options, functional options, performance limits, control parameters, compatible bloodlines, and language options remain unchanged from the predicate device, the 2008K.
Also, an article in the February 2011 issue of Renal Business Today takes “A Peek Inside the New Fresenius 2008T”. From the article it’s clear that the change to the user interface refers to the addition of an integrated medical information system that will work with the software from any of the major vendors in the dialysis MIS industry.
Recent feedback on RenalWeb suggests that the 2008T is now readily available for trial or purchase. How many of you here have conducted trials with this machine in your dialysis center?

In 2010, B. Braun introduced its Adimea System for use with the Dialog Plus dialysis machine. The Adimea System provides continuous online monitoring of dialysis dose during the patient treatment.
It utilizes the principles of light absorption in matter for determining the reduction in the concentration of urinary excreted substances in the dialysate drain. A light source transmits ultraviolet light through the dialysate.
The particles contained in the dialysate, which were removed from the plasma during dialysis, absorb the light. This absorption is measured by a sensor.
According to Braun, ultraviolet absorption measurements can be used to determine dialysis dose because of the close linear correlation between the measured UV absorption signal and the urea concentration in the dialysate.
It utilizes the principles of light absorption in matter for determining the reduction in the concentration of urinary excreted substances in the dialysate drain. A light source transmits ultraviolet light through the dialysate.
The particles contained in the dialysate, which were removed from the plasma during dialysis, absorb the light. This absorption is measured by a sensor.
According to Braun, ultraviolet absorption measurements can be used to determine dialysis dose because of the close linear correlation between the measured UV absorption signal and the urea concentration in the dialysate.

Moving on from dialysis machines, let’s take a quick look at Concentrate Mixing Systems.
In 1989, Renal Systems, a division of Minntech, introduced the Renapak. It used a venturi-based suction wand to automatically draw up dry powder concentrate from a drum into a 100 gallon tank where it was re-circulated until mixed. The formulation labels on the powder drums all had stated conductivity values. The completed liquid concentrate batches could be QC tested using conductivity meters specifically designed for that purpose.
The Renapak 2 and Rockwell systems were introduced in 2000.
It’s my understanding that the dry powder formulations no longer have conductivity values listed on the labels. It’s my opinion that this is a step backward, as it eliminates the ability to do an easy, accurate QC test of the mixed concentrate solutions.
By the way, does anyone recognize the guy in the Renapak picture on the left?
It’s Michael Honstein of RPC when he worked for Southwest Kidney Institute in Arizona. Don’t tell him I said so, but I think his hair is a lot more gray these days.
In 1989, Renal Systems, a division of Minntech, introduced the Renapak. It used a venturi-based suction wand to automatically draw up dry powder concentrate from a drum into a 100 gallon tank where it was re-circulated until mixed. The formulation labels on the powder drums all had stated conductivity values. The completed liquid concentrate batches could be QC tested using conductivity meters specifically designed for that purpose.
The Renapak 2 and Rockwell systems were introduced in 2000.
It’s my understanding that the dry powder formulations no longer have conductivity values listed on the labels. It’s my opinion that this is a step backward, as it eliminates the ability to do an easy, accurate QC test of the mixed concentrate solutions.
By the way, does anyone recognize the guy in the Renapak picture on the left?
It’s Michael Honstein of RPC when he worked for Southwest Kidney Institute in Arizona. Don’t tell him I said so, but I think his hair is a lot more gray these days.
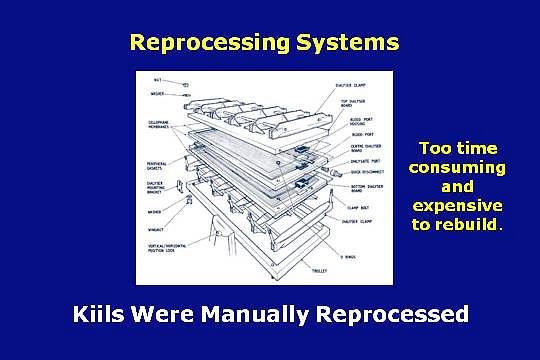
The origin of dialyzer reprocessing can be traced to the early years of dialysis when there was a need to dialyze an ever increasing number of patients, during a time when there was limited availability of non-disposable dialyzers.
To save time and money, manual procedures were developed to reprocess the dialyzers, such as the Kiil board.
To save time and money, manual procedures were developed to reprocess the dialyzers, such as the Kiil board.

After the introduction of the Cordis-Dow hollow fiber dialyzer — to reprocess these dialyzers — a number of inventive and pioneering dialysis centers developed their own manual reuse system, like the one shown here.
The manual systems consisted of a series of connectors, pipes, valves, and tanks.
Many of the dialysis centers that were among the first to develop manual reuse systems, were also the researchers who first studied the safety and efficacy of reprocessing hollow fiber dialyzers. A few that come to mind include Dr. Deane’s group at Manhattan Kidney Center, Dr. Ogden’s group at Dialysis Foundation of Southern Arizona, and Dr. Shapiro’s group at the Regional Kidney Disease Program in Minneapolis. The RKDP group based in Minneapolis ultimately became a part of TRC, which is now DaVita.
The manual systems consisted of a series of connectors, pipes, valves, and tanks.
Many of the dialysis centers that were among the first to develop manual reuse systems, were also the researchers who first studied the safety and efficacy of reprocessing hollow fiber dialyzers. A few that come to mind include Dr. Deane’s group at Manhattan Kidney Center, Dr. Ogden’s group at Dialysis Foundation of Southern Arizona, and Dr. Shapiro’s group at the Regional Kidney Disease Program in Minneapolis. The RKDP group based in Minneapolis ultimately became a part of TRC, which is now DaVita.

In the late 1970s, United Medical introduced the Lixivitron. It was the first fully automated dialyzer reprocessing system. Dialysis centers leased the machine and paid a fee for each dialyzer reprocessed.

In the early 1980s, automated reprocessing systems were introduced by Mesa Laboratories, Renal Systems, and Seratronics. Originally, all three machines used formaldehyde as the final germicide that was delivered to the dialyzer for storage.
After 2 to 3 years of offering a formaldehyde version of the Renatron, Renal Systems modified the Renatron to accept Renalin, a peracetic acid germicide. The rapid success of the Renalin Renatron resulted in a quick discontinuation of the formaldehyde version. The blue Renalin Renatron shown here evolved from a one-program, discrete electronic version into a microprocessor-based version that allowed the operator to select from three different reprocessing programs.
For many years, the Echo and DRS-4 continued with formaldehyde or glutaraldehyde as the final germicide.
After 2 to 3 years of offering a formaldehyde version of the Renatron, Renal Systems modified the Renatron to accept Renalin, a peracetic acid germicide. The rapid success of the Renalin Renatron resulted in a quick discontinuation of the formaldehyde version. The blue Renalin Renatron shown here evolved from a one-program, discrete electronic version into a microprocessor-based version that allowed the operator to select from three different reprocessing programs.
For many years, the Echo and DRS-4 continued with formaldehyde or glutaraldehyde as the final germicide.

All three of the automated reprocessing systems evolved to include data management systems and other features. When the standalone data management system was introduced, the color of the Echo system was changed to white.
For the Renatron, its upgrade was much more involved ... in that the data management system called Renalog, was actually electronically tied to one or more Renatrons to automatically import information from the machines.
In addition, many other features were added, the color was changed to beige, and it was renamed as the Renatron II System.
Its combination of selectable reprocessing programs, size flexibility, and successful use of peracetic acid, ultimately led to the Renatron capturing most of the market for dialyzer reprocessing machines.
For the Renatron, its upgrade was much more involved ... in that the data management system called Renalog, was actually electronically tied to one or more Renatrons to automatically import information from the machines.
In addition, many other features were added, the color was changed to beige, and it was renamed as the Renatron II System.
Its combination of selectable reprocessing programs, size flexibility, and successful use of peracetic acid, ultimately led to the Renatron capturing most of the market for dialyzer reprocessing machines.

In the first years of decade 2000, two additional automated dialyzer reprocessing systems were introduced: The Alcavis ARM and the HDC Maky.
In 2008, Alcavis and HDC merged. The current Web site for the combined company no longer shows the ARM machine. It may mean that they have made a decision to de-emphasize it in favor of the Maky machine.
While the basic Renatron II system design is many years older than the ARM and Maky, the Renatron II continues to be the leading dialyzer reprocessing system. It will be interesting to see what happens if the new CMS bundled payment results in a significant increase in dialyzer reprocessing for the small- and mid-size dialysis organizations.
In 2008, Alcavis and HDC merged. The current Web site for the combined company no longer shows the ARM machine. It may mean that they have made a decision to de-emphasize it in favor of the Maky machine.
While the basic Renatron II system design is many years older than the ARM and Maky, the Renatron II continues to be the leading dialyzer reprocessing system. It will be interesting to see what happens if the new CMS bundled payment results in a significant increase in dialyzer reprocessing for the small- and mid-size dialysis organizations.

The reprocessing machines shown on the left side and top right are all produced in Asian countries. In the lower right corner is the ClearFlux system from Novaflux Technologies, a company based in Princeton, New Jersey. The ClearFlux system received FDA 510k clearance in November of 2010.
As of this date (March 2011), none of the other machines shown here have FDA 510k clearance and are not established in the United States.
As of this date (March 2011), none of the other machines shown here have FDA 510k clearance and are not established in the United States.

Formaldehyde and glutaraldehyde were the primary dialyzer reprocessing germicides originally used in the United States. Also, for a very short period of time, there was a chlorine dioxide based germicide called ReNu D. Because it dramatically altered some dialyzer membranes, it was quickly pulled from the market.
After the introduction of Renalin in the 1980s, other equivalent peracetic acid germicides were ultimately introduced, including Puristeril from Fresenius, Peracidin from HDC Medical and Micro-X from RPC.
The peracetic acid germicides have been so successful that only a handful of dialysis centers still use formaldehyde and glutaraldehyde to reprocess dialyzers.
After the introduction of Renalin in the 1980s, other equivalent peracetic acid germicides were ultimately introduced, including Puristeril from Fresenius, Peracidin from HDC Medical and Micro-X from RPC.
The peracetic acid germicides have been so successful that only a handful of dialysis centers still use formaldehyde and glutaraldehyde to reprocess dialyzers.

Anyone recognize any of these reprocessing veterans?
In the photo on the left is: Mike Kallay — who is still with Minntech (sales manager for the western states); Cathy Hicks — the former Clinical Educator at St. Joe’s in Orange, California; and the late Dominick Gentile — former Medical Director at St. Joe’s in Orange.
In the photo on the right, in the lab coat, is: Mike Fischer – the former administrator at the original Southwest Kidney Institute in Tucson.
Anyone know who that guy is next to Mike Fischer in the photo on the right?
In the photo on the left is: Mike Kallay — who is still with Minntech (sales manager for the western states); Cathy Hicks — the former Clinical Educator at St. Joe’s in Orange, California; and the late Dominick Gentile — former Medical Director at St. Joe’s in Orange.
In the photo on the right, in the lab coat, is: Mike Fischer – the former administrator at the original Southwest Kidney Institute in Tucson.
Anyone know who that guy is next to Mike Fischer in the photo on the right?

If you didn’t recognize the people on the last slide, perhaps you will recognize these reprocessing dinosaurs.
In the picture on the left is: Doug Luehmann – Doug is now retired but is still well known for his many contributions on the technical side of dialysis. And Dave Squillace — of Mayo Clinic
In the picture on the right is: Wayne Carlson — of Minntech; and Cindy Rios — a long-time NANT contributor.
In the picture on the left is: Doug Luehmann – Doug is now retired but is still well known for his many contributions on the technical side of dialysis. And Dave Squillace — of Mayo Clinic
In the picture on the right is: Wayne Carlson — of Minntech; and Cindy Rios — a long-time NANT contributor.
Okay. Let’s move on to water systems.
In many — perhaps most — cases, the water used to make up dialysate for the earliest dialysis treatments was tap water, or untreated water by today’s standards.
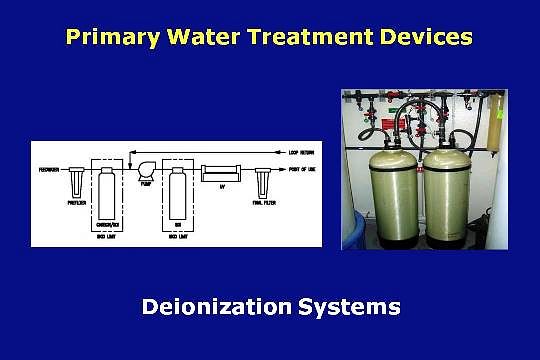
Eventually de-ionization based systems began to be used to remove contaminants from the water.
In many — perhaps most — cases, the water used to make up dialysate for the earliest dialysis treatments was tap water, or untreated water by today’s standards.
Eventually de-ionization based systems began to be used to remove contaminants from the water.

As time passed, patient safety-related problems became a big issue with de-ionization-based systems. As a result, reverse osmosis membrane based systems began to be introduced. Some dialysis centers built their own RO systems, including a central RO machine. If memory serves me, RKDP in Minneapolis built their own complete RO system during the late 1970s or early 1980s.
Among the earliest commercial ROs was the Model 19C shown on the left. It was manufactured by Osmonics, a company based in the Minneapolis area.
Among the earliest commercial ROs was the Model 19C shown on the left. It was manufactured by Osmonics, a company based in the Minneapolis area.

Like the dialysis machine manufacturers, the water system manufacturers also have experienced a great deal of change due to mergers or acquisitions.
In 1994, Osmonics introduced their Model 23G which continues to be a popular RO today.
In 1999, Osmonics acquired ZyzaTech, a dialysis water treatment device manufacturer based in Kent, Washington.
In 2003, GE Water acquired Osmonics. Also in 2003, Cantel (the parent company of Minntech), acquired Mar Cor Purification.
In 2007, Mar Cor acquired GE Water.
Since 2007, Mar Cor has acquired Biopure (a prominent Canadian water treatment company) and other smaller regional U.S. water-treatment companies. In October of last year (2010), Mar Cor acquired the rights to Gambro’s U.S. water-treatment products.
In 1994, Osmonics introduced their Model 23G which continues to be a popular RO today.
In 1999, Osmonics acquired ZyzaTech, a dialysis water treatment device manufacturer based in Kent, Washington.
In 2003, GE Water acquired Osmonics. Also in 2003, Cantel (the parent company of Minntech), acquired Mar Cor Purification.
In 2007, Mar Cor acquired GE Water.
Since 2007, Mar Cor has acquired Biopure (a prominent Canadian water treatment company) and other smaller regional U.S. water-treatment companies. In October of last year (2010), Mar Cor acquired the rights to Gambro’s U.S. water-treatment products.

The Biopure and ZyzaTech ROs on this slide were introduced in the mid-to-late 1990s. As a result of the acquisitions pointed out in the last slide, both devices are now part of the Mar Cor line of ROs.

The Gambro WRO and the U.S. Filter Med-RO were introduced in 1998. Heated water disinfection is a significant feature of the Gambro WRO.

RO machines introduced more recently include the Isopure MD series and the Better Water Matrix.

To conclude this review, it’s important to ask the following question: Can dialysis devices ever be more important than the people that operate and maintain them?
I submit that you are Number One. A dialysis device is only as good as the people that operate and maintain it.
To that end, I would like to express my admiration and regard to each of you for your significant contribution to the past, present, and future of dialysis — and I thank you for participating in this session.
Please fire away with any questions or comments you have.
I submit that you are Number One. A dialysis device is only as good as the people that operate and maintain it.
To that end, I would like to express my admiration and regard to each of you for your significant contribution to the past, present, and future of dialysis — and I thank you for participating in this session.
Please fire away with any questions or comments you have.
